Why Is Ethanol More Soluble in Water Than Ethane
Water and surface tension is going to be based on the force of confusion between the molecules or the attraction between the mo Limited Time Offer Unlock a free month of Numerade by answering 20 questions on our new app StudyParty. Ethane which is composed of carbon and hydrogen only has no polar group and is not water -.

Solved Complete The Sentences Identifying Which Compound In Chegg Com
The energy of hydration offsets this energy input in the case of ethanol but not ethene.
. Why is ethanol more soluble in water than hexanol. Solubility of Ethanol in Water Explain why ethanol CH 3 CH 2 OH is more soluble in water than is ethane CH 3 CH 3 Answer Field 2. Ethanol is polar Ethane is not.
Properties of a Buffer The amino acid glycine is often used as the main ingredient of a buffer in biochemical experiments. In other words ethanol is soluble in water because it is a polar solvent. Now its obvious the more bulkier the group around oxygen the less space around oxygen to form this bond.
Because it does have a higher polarity compared with ethane. The amino group of. We know that alcohols form more number of hydrogen bonds than alkyl halides.
And ethane only offers the possibility of weaker dispersion forces between molecules and has a boiling point of 89 C. Ethanol is more soluble in water because the hydroxyl group on the end of alcohol forms hydrogen bonds with water moleculeswhile ethane is hydrophobic and causes the solution to be highy ordered. The ethanol -OH group can hydrogen bond with water.
So polarization is weak to make strong intermolecular forces between water molecules. Furthermore the melting and boiling temperatures of ethanol are comparatively higher than that of ethane because ethanol can form strong bonds such as hydrogen bonds between molecules which. When considering the chemical composition of two compounds ethane contains carbon and hydrogen atoms while ethanol has carbon hydrogen and oxygen atoms.
Sikringbp and 2 more users found this answer helpful. Ethanol is soluble in water because the polarity of its hydroxyl group is stronger than the nonpolarity of its two carbon chain. Ethanol is more soluble in water compared to hexanol as the non polar hydrocarbon chain in hexanol is longer than the non polar hydrocarbon chain in ethano.
So Ethanol being an alcohol is more soluble in. In alcohols with more than five carbons in their chain the repulsive forces between the nonpolar chain and polar water do not allow the two to mix according to Solubility of Things. Alcohol molecules can hydrogen bond to each other and to water molecules.
Solubility of Ethanol in Water Explain why ethanol CH3CH2OH is more soluble in water than is ethane CH3CH3. Methane ethane propane other alkanes are not soluble in water. Ethanol is more soluble in water because the hydroxyl group on the end of alcohol forms hydrogen bonds with water moleculeswhile ethane is hydrophobic and causes the solution to be highy ordered.
Ethanol has a polar OH group which hydrogen bonds to water. Why is ethanol more soluble in water than ethane is. This explains their solubility in water.
Haricharan Raghunathan added an answer on 13116. Ethanol is more soluble in water because the hydroxyl OH group on the end of the alcohol can form Hydrogen bonds with the water molecules where ethane is hydrophobic and causes the solution to. Ethanol has an OH group and only 2 carbon.
General Organic and Biochemistry 1st Edition Edit edition Solutions for Chapter 9 Problem 53CQ. Ethanol is soluble in water primarily because of the presence of -OH group that allows or enables it to form hydrogen bonds with water molecules. At room temperature ethanol is a liquid whilst ethane is a gas.
Hence C H X 3 O H is more soluble in water than C X 2 H X 5 O H. After all C X 2 H X 5 group is more soluble than C H X 3 group. Explain why ethanol CH3CH2OH is more soluble in water than is ethane CH3CH3.
For solubility in water which incidentally a polar solvent the compound which forms more number of hydrogen bonds is more soluble. Why is ethanol soluble in water. Calculation of p H from Hydrogen Ion Concentration What is the p H of a solution that has an H concentration of.
And the hydrogen attached to oxygen of ethanol makes a hydrogen bond with the oxygen of water. James Armstrong Rent Buy. Ethene propene are also not soluble in water.
It takes energy to take molecules of the substance dissolved appart. Which makes ethanol soluble. Why ethanol is more soluble in water than ethane.
Haricharan Raghunathan answered this. On the other hand ethyl alcohol has a boiling point of 78 C and this reflects the influence of hydrogen- bonding as a binding force between molecules. Except that as many others have stated both methanol and ethanol are infinitely miscible in water.
Acetylene propyne and so do not dissolve in water. This makes it possible to overcome the IMAFs of water. Whereas ethanes weak london dispersion bonds would not be able to overcome waters IMAFs making it insoluble.
The dissolving of ethanol is thus energetically favored at large amounts whereas ethane-water mixtures tend to be most stable when in separation. Ethanol is more soluble in water than ethane due to the fact that ethanol has hydrogen bonding. The ethanol OH group can hydrogen-bond with water The ethanol OH group can hydrogen-bond with water.
Alcohol molecules can hydrogen bond to water molecule so alcohols are soluble in water. Therefore alkane alkene and alkyne are not soluble in water. Why Ethanol is soluble in water while ethane is not.

Solved Explain The Fact That Ethanol Ch 3 Ch 2oh Is More Chegg Com

Answered 35 38 Ethanol Is Freely Soluble In Bartleby
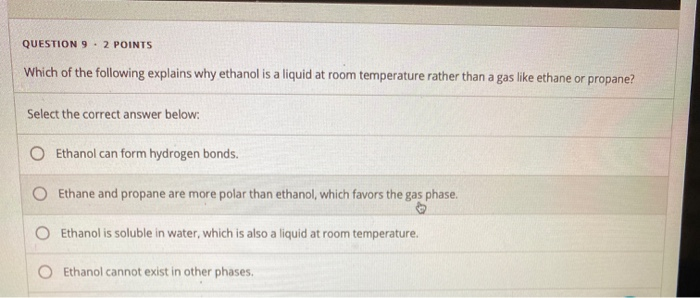
Solved Question 9 2 Points Which Of The Following Explains Chegg Com
Why Is Ethanol More Soluble In Water Than Ethyl Ethanoate Quora
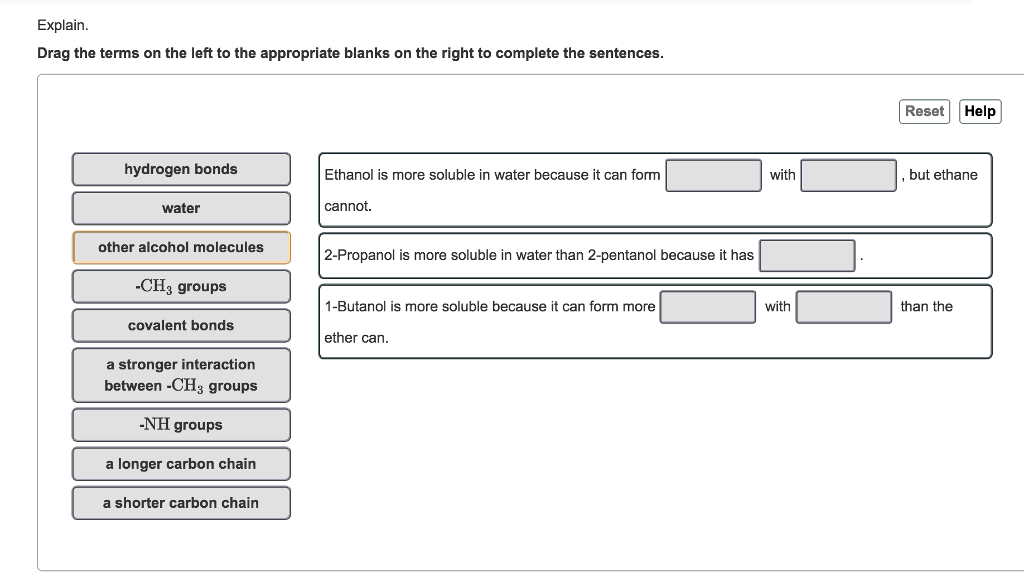
Solved Explain Drag The Terms On The Left To The Chegg Com

Solved Match The Words In The Left Column To The Appropriate Chegg Com
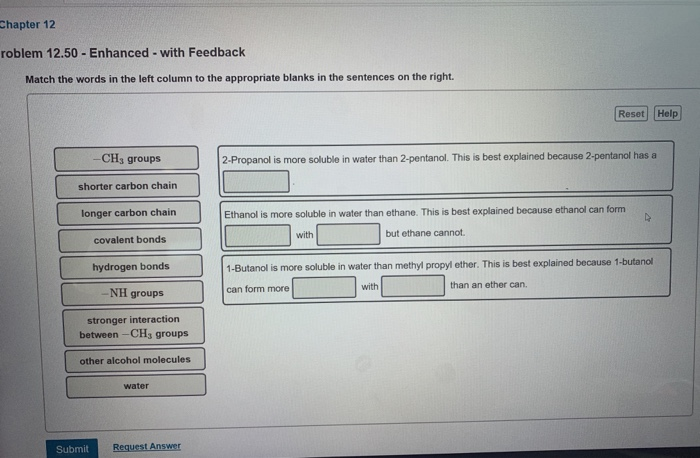
Solved Chapter 12 Roblem 12 50 Enhanced With Feedback Chegg Com

Solved Problem 1 Explain Why Ethanol Ch Ch Oh Is More Chegg Com

Solved A Technician In A Hospital Laboratory Obtained A 10 0 Chegg Com

Account For The Following Ethanal Is More Soluble In Water Than Ethane But Less Than The Ethanol From Chemistry Aldehydes Ketones And Carboxylic Acids Class 12 Haryana Board English Medium
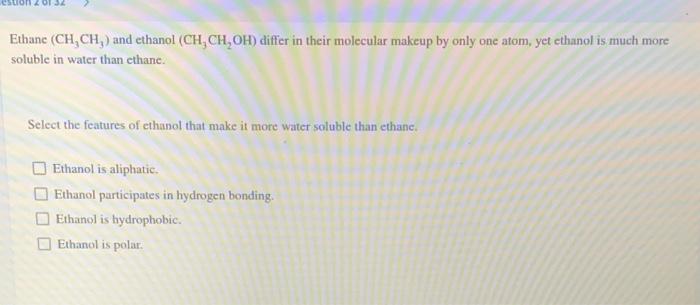
Solved Ethanc Ch Ch And Ethanol Ch Ch Oh Differ In Chegg Com
Why Is Ethanol Soluble In Water Quora

Solved Using Suitable Diagrams Explain Why Ethanol Is Soluble In Water While Ethane Is Not Definc The Tcrm Solubility

Solved Homework 1 Name Each Problem Is Worth 10 Points Chegg Com

Solved 1 The Interactions Between Biomolecules Are Often Chegg Com
Why Is Ethanol More Soluble In Water Than Methanol Quora

Solved Why Is Ethanol Ch3ch20h More Soluble In Water Than Chegg Com
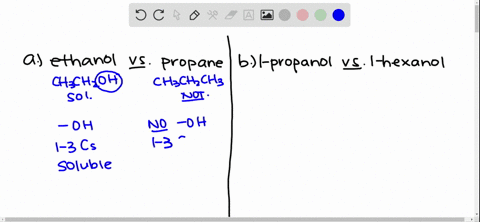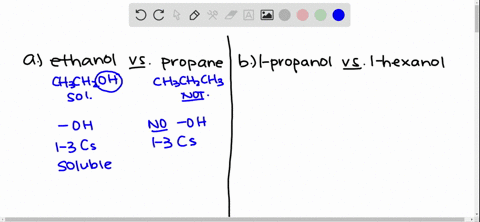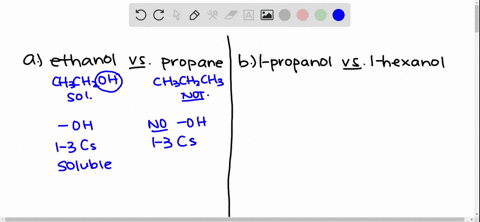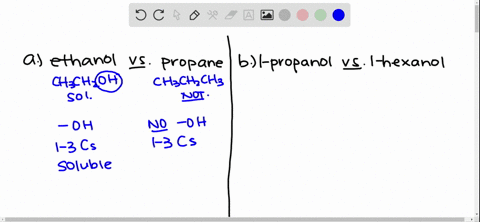
Solved Give An Explanation For The Following Observations A Methanol Is Soluble In Water But Ethane Is Not B 2 Propanol Is Soluble In Water But 1 Butanol Is Only Slightly Soluble
Comments
Post a Comment